Mystery of the Elements
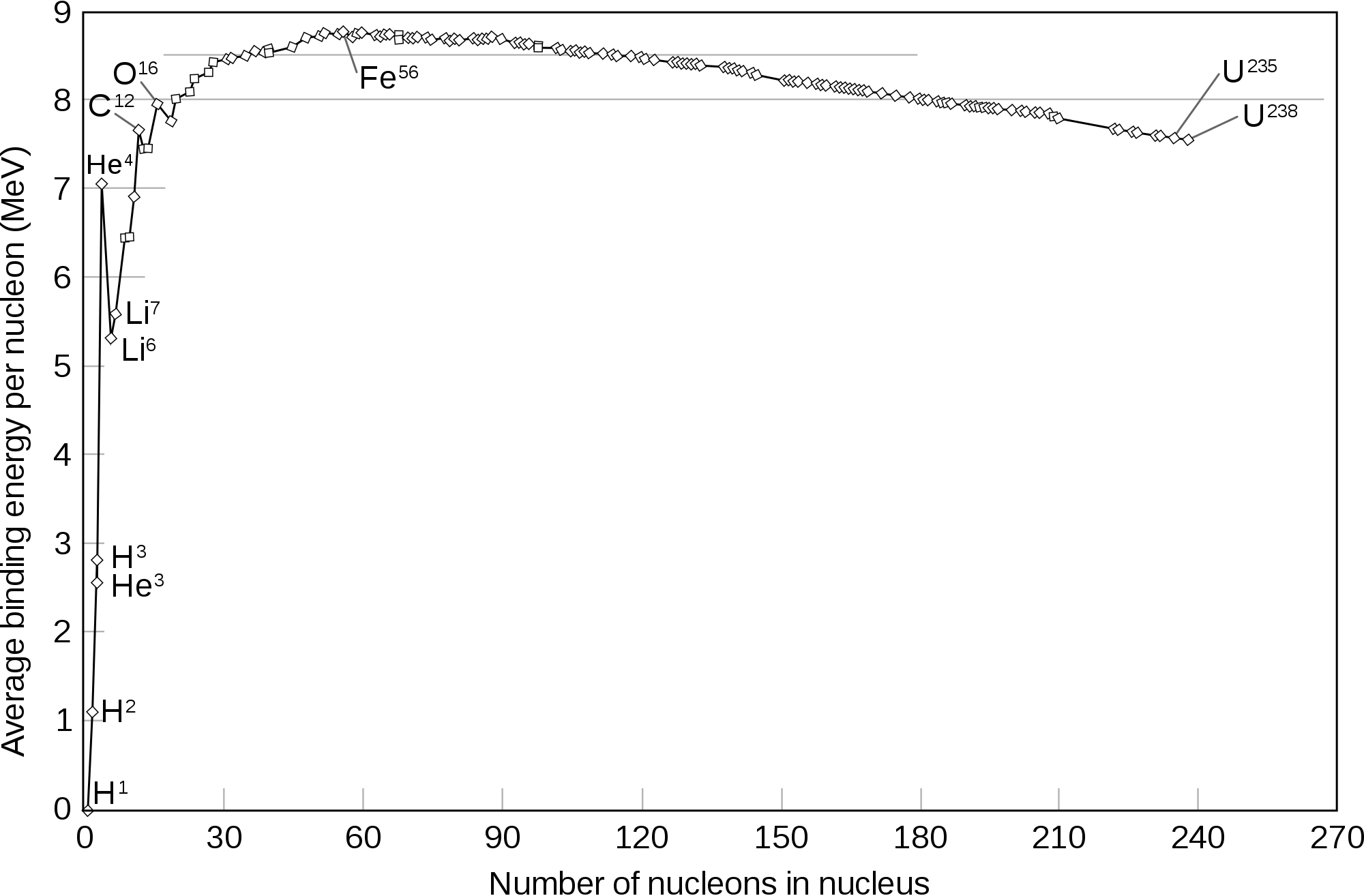
Recognize this curve? It is the binding energy per nucleon of the most common isotope of every element in the periodic table. From this, it is clear that iron and nickel have the most stable nuclei. Their binding energy is around 8.8 MeV per nucleon. That's 50000000000000 joules of energy required to split the nucleons of a mole of iron. This is around 350 times the energy released by both the atomic bombs dropped on Japan combined! So this is proof enough that these elements are pretty stable. It makes sense that the heavier elements such as uranium, on decomposition give elements to the left of the curve, tending to more stability. This is a process called fission. The lighter nuclei too, join together or fuse together to form heavier nuclei in the process called fusion. They too, tend towards stability and move towards iron in the curve. Since the elements on both extremes of the curve tend to the stable middle sections, how on Earth were the heavy elements formed? Does this mean nature breaks its untold general rule - everything loves to be in a stable state?
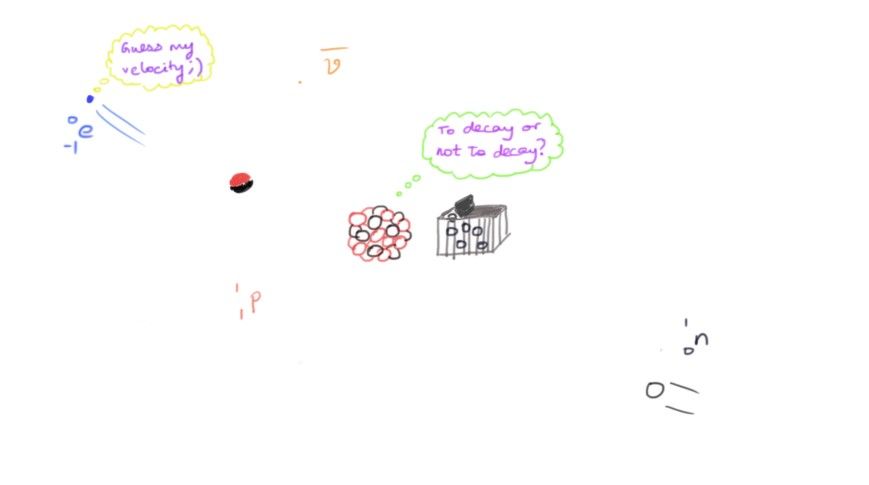
When I attempted to answer this question, my first explanation was beta decay. My thought process went like the lighter nuclei increases its atomic number to a largish-value and then attempts to gain enough neutrons to balance it... But this didn't seem convincing, even to me. But this was partly right.
My second explanation was better, fuse the stable nuclei with a huge amount of energy in such a way they are forced to join. The source of energy - stars. Not our sun, it's too small, but in the bigger ones with more fuel. This too was only partially correct.
The correct answer, which I found after some research was actually a mixture of these two answers - neutron capture. Thermonuclear fusion (my second idea) fails to overcome the repulsion between two nuclei, but a neutron doesn't undergo repulsion. And neutrons are absorbed and then the beta decay occurs to give the elements.
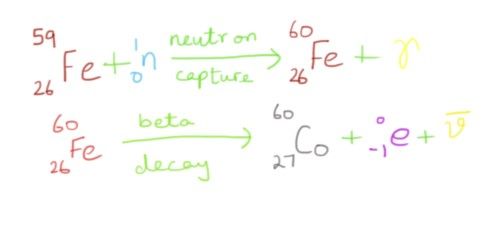
The above illustration shows how cobalt, a 'heavier' element is formed. We move out of the barrier set by iron, and its high energy standards with this nifty technique. In this same way, gold can be converted to mercury, cadmium to indium, strontium to yttrium and so on.
But what if I have a lot of neutrons to waste? Another pathway, called the rapid neutron capture process, or simply as the r-process occurs. By a lot of neutrons, I mean almost one and a half moles of free neutrons in one cubic centimeter. All this for a temperature of around a billion degrees. These are huge numbers beyond comprehension, and they need a drastic location for this to take place, not to mention the enormous amount of energy required. This location: a supernova explosion or a neutron star. The nucleus capturing a neutron takes in a lot of neutrons very quickly, there is almost no time for the nucleus to beta decay to form an element. Only after the nucleus becomes really unstable is when it finally beta decays to form an element with a higher atomic number.

Another way to create these elements is by the slow neutron capture process, or the s-process. Here, a nucleus undergoes normal neutron capture to form a heavy isotope. This happens till the isotope becomes unstable. Beta decay now occurs to form a stable new element. This process is 'slow' because there is enough time for a nucleus to decay if it is unstable before capturing another neutron.
The r-process and the s-process together account for almost all the elements after iron in the periodic table. So far so good. There's an unsightly gap at atomic number 43 though. This seat was to be occupied by technetium, the first element synthesized artificially by humans in 1936. There are no stable isotopes of technetium, which explains this absence. There are very minute quantities of this element which happens to be a fission product of uranium. It can also be formed by neutron capture of molybdenum. Similarly, promethium, astatine, neptunium, and plutonium were discovered through synthesis before being found in nature.
Just when the world breathed a sigh of relief after Russia and the US put away their Little Boys and their Tsars, turns out their scientists had been busy playing with their fuel and pummeling big balls with little ones in their labs. And lo and behold, the greatest of miracles, some short-lived merged balls were produced! And then they began to fight over naming them.